TRANSMIT
TRANSlating the role of Mitochondria in Tumorigenesis
NETWORK AND PARTNERS
The consolidation of the knowledge that cancer is not only a genetic, but also a metabolic disease, has led scientists to investigate the intricate metabolic plasticity that transformed cells must undergo to survive the adverse tumor microenvironment conditions, and the contribution of oncogenes and tumor suppressors in shaping metabolism. In this scenario, genetic, biochemical and clinical evidences place mitochondria as key actors in cancer metabolic restructuring, not only because these organelles have a crucial role in the energy and biosynthetic intermediates production but also because occurrence of mutations in metabolic enzymes encoded by both nuclear and mitochondrial DNA has been associated to different types of cancer. TRANSMIT aims to dissect the metabolic remodeling in human cancers, placing the focus on the role of mitochondria and bridging basic research to the improvement/development of therapeutic strategies. Further, TRANSMIT fosters the communication of this emerging field to the patients and their families. To these aims, TRANSMIT will create a network of seven different countries, among which world-leading basic science and clinical centers of excellence, several industrial partners with up-to-date omics technologies, as well as non-profit foundations and associations who care for cancer patients. By creating the critical mass of scientific excellence, TRANSMIT will allow to transfer the current knowledge into the wide field of cancer research, translating scientific and technical advances into the education and training of eleven Early Stage Researchers. TRANSMIT will implement training-through-research dedicated to unravel the metabolic features of cancer, as well as to provide a full portfolio of complementary skills through the creation of a network of basic, translational and industrial laboratories, devoted to a multidisciplinary/multi-sectorial education of young scientists.
TRANSMIT is a multi-partner Marie Curie ITN project, funded within the frame of H2020 program, that intends to mobilize the critical mass of expertise, by linking partners from 7 different countries, among which 7 world-leading basic science labs, 3 private SMEs, 4 additional Associate Partners (3 SMEs and 1 Foundation) that will provide for all trainees the most fertile training environment.
List of Beneficiaries
| Consortium Member | Short Name | Country | Department | Scientist in charge |
| University of Bologna | UNIBO | Italy | Pharmacy and Biotechnologies | Anna Maria Porcelli |
| Université catholique de Louvain | UCL | Belgium | Pharmacology and Therapeutics | Pierre Sonveaux |
| Oroboros Instruments GmbH | OROBOROS | Austria | Research & Development | Eric Gnaiger |
| Justus-Liebig-University of Giessen | JLU | Germany | Veterinary Physiology and Biochemistry | Sybille Mazurek |
| Gemeinnutzige Salzburger Lande Skliniken Betriebsgesellschaft | SALK | Austria | Pediatrics University Hospital Salzburg | Barbara Kofler |
| Karolinska Institute | KI | Sweden | Oncology-Pathology R8 | Maria Shoshan |
| The Chancellor, Masters and Scholars of the University of Cambridge | UNICAM | UK | Medical Research Council Cancer Unit | Christian Frezza |
| Universite de Bordeaux | UBOR | France | Rare Diseases Genetics and Metabolism | Rodrigue Rossignol |
| Biocrates Life Sciences AG | BIOCRATES | Austria | Research & Development | Guido Dallmann |
| AvantiCell Science Ltd UNTIL JANUARY 2020 | ACS | UK | Research & Development | Colin Wilde |
| Consortium Member | Short Name | Country | Department | Scientist in charge |
List of Partners Organizations
| Partner Organization | Short name | Country | Field |
| Fondazione Umberto Veronesi | FUV | Italy | Communication |
| INNOVA SPA | INNOVA | Italy | Training |
| CEUB.SOC.CONS.ARL | CEUB | Italy | Training |
| Dynamo Academy Srl Impresa Sociale | Dynamo Academy | Italy | Training |
| MEDIZINISCHE UNIVERSITAT INNSBRUCK | MUI | Austria | Training |
| Partner Organization | Short name | Country | Field |
WORK PACKAGES DESCRIPTION
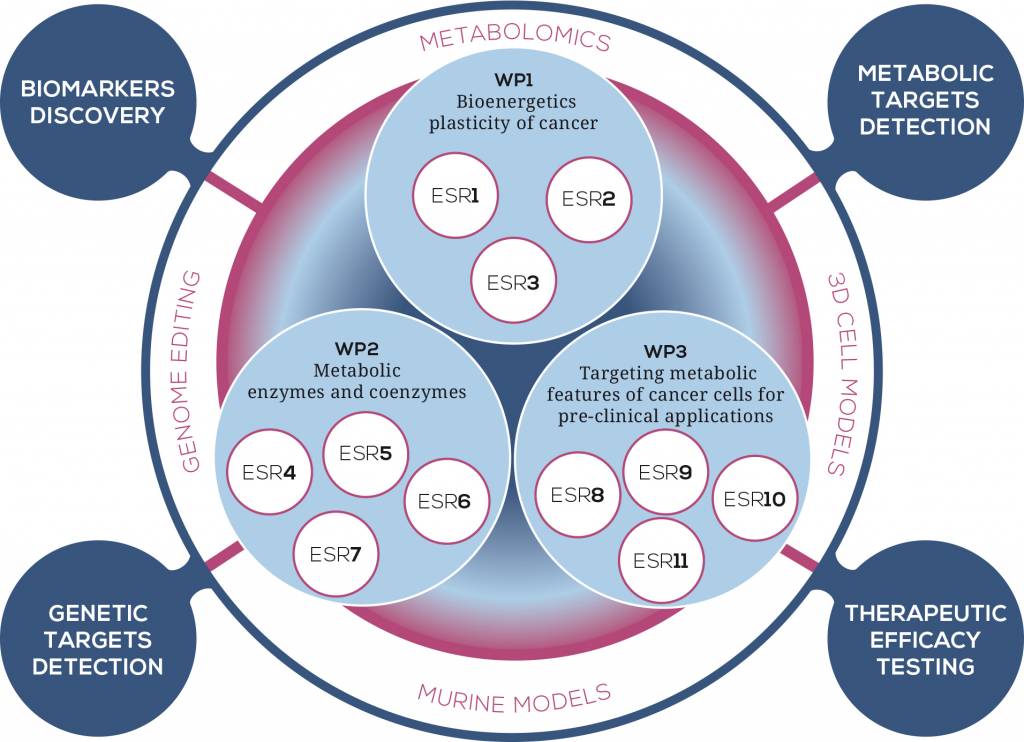
WP1 – Bioenergetics plasticity of cancer
The field of energy metabolism dramatically progressed in the last decade, owing to a large number of cancer studies, as well as fundamental investigations on related transcriptional networks and cellular interactions with the microenvironment. The concepts of bioenergetic plasticity and metabolic flexibility were raised in studies showing the ability of cancer cells to remodel the biochemical pathways of energy transduction in response to changes in the microenvironment or following oncogenes activation or tumor suppressor inactivation .Different types of metabolic remodeling have been reported, and it is crucial to mention here that not all cancer cells conform to the Warburg effect, and that some cancer cells display an opposite phenotype, i.e., with enhancement of the OXPHOS system and activation of glutaminolysis. Several modes of glutamine utilization have been described but little is known on the metabolic remodeling that utilizes fatty acids as carbon substrates, or aminoacids. Metabolic flexibility relies on the rewiring of existing metabolic pathways, with powerful analytical tools needed to decipher the concerted change in a large number of biochemical reactions involved in tumor metabolism. UBOR, leader of this WP, will develop a metabo-proteomic evaluation of the large-scale changes in metabolic network in order to define and analyze the functional impact by means of bioinformatic tools. By improving high-resolution respirometry techniques OROBOROS will identify mitochondrial metabolic biomarkers underlying cell transformation. Finally, JLU will dissect the interplay between different energetic and biosynthetic pathways involved in metabolic reprogramming.

Rodrigue Rossignol, UBOR
WP Leader
Project: Bioenergetics of lung tumors

Sybille Mazurek, JLU
Principal Investigator
Project: Coordination of glutaminolysis and glycolysis in cancer cells

Erich Gnaiger, OROBOROS
Principal Investigator
Project: Cell ergometry and mitochondrial metabolic biomarkers in cancer
WP2 – Metabolic enzymes and coenzymes
Over the past decade a large body of work has demonstrated that oncogenes and tumor suppressors play a key role in regulating the metabolic transformation of cancer cells and in directing the use of glucose, glutamine, and other nutrients to the generation of lipids, nucleic acids, and proteins for dividing cells. Mitochondria are key actors in the metabolic reprogramming of cancer cells, and several oncogenes and tumor suppressors exert their metabolic function via modulation of mitochondrial processes. The key role of mitochondria in cancer is further corroborated by the finding that mitochondrial enzymes, when mutated, can predispose to hereditary and sporadic forms of cancer. For instance, mutations of the mitochondrial housekeeping genes such as succinate dehydrogenase (SDH), fumarate hydratase (FH) and isocitrate dehydrogenase-2 were identified in different types of cancer, suggesting that, in some circumstances, mitochondrial dysfunction sufficient to drive tumorigenesis. Two of these genes, SDH and FH, are classified as tumor suppressors, as affected individuals inherit one mutated copy of the relevant gene, whereas the tumors exhibit loss of the wild-type allele following a somatic ‘second event’ in keeping with Knudson’s classic two-hit hypothesis. Additionally, a recent bulk of evidence points to a role for mutations in mtDNA in driving cancer progression and adaptation, indicating that respiratory complex proteins, which are also metabolic enzymes, have a full potential to be annotated as tumor suppressors or oncogenes, or even play a double-edged role according to the type and load of mutations. Investigations of the mechanisms of action of mitochondrial defective enzymes ultimately revealed new players in the field of oncology, namely the oncometabolites, i.e. small metabolites, such as fumarate and succinate, that, when accumulated as a consequence of mitochondrial dysfunction, induce profound genetic and epigenetics changes that predispose to cancer cell transformation. Indeed, a mechanistic gap exists between the finding of cancer-associated genetic lesions and tumor progression, which this WP intends to fill in with an in-depth investigation of the regulators and pathways which are activated following mutations in metabolic enzymes, and that ultimately lead to a pro-tumorigenic metabolic rewiring. To this aim, UCAM will investigate how the loss of the mitochondrial tumor suppressor FH predisposes to cancer transformation. UNIBO will dissect how respiratory complex I (CI) can modulate metabolic and hypoxia adaptation. KI will tackle how mitochondrial biogenesis regulators, upstream of metabolic enzymes, may impact on cancer progression and adaptation. Lastly, BIOCRATES will improve different technologies to determine the contribution of cofactors in cancer metabolic reprogramming.

Giuseppe Gasparre, UNIBO
WP Leader
Project: Mitochondrial complex I-driven regulation of the hypoxic response in cancer cells

Christian Frezza, UNICAM
Principal Investigator
Project: Fumarase and fumarate: epigenetic modifications in FH-deficient tumors

Maria Shoshan, KI
Principal Investigator
Project: Roles of mitochondrial biogenesis enzymes in regulation of chemoresistance
WP3 – Targeting metabolic features of cancer cells for pre-clinical applications
One of the encountered hurdles in treatment of cancer is the partial or complete resistance of certain tumors to conventional therapeutic interventions. Furthermore, it is well accepted that cancer manifests into many types, depending on the tissue affected, and that both environmental and genetic factors influence the metabolic, hypoxic and vascularization features of this disease. Thus, one of the most important challenges in medicine is the development of novel and more effective therapies to improve survival of cancer patients. The peculiar metabolic profile of cancer may be an ideal target for therapeutic interventions that selectively hit cancer cells with minimal residual systemic toxicity. Within TRAMSMIT we will attempt to understand the mechanisms leading to altered tumor cell metabolism and its consequences to open up new therapeutic windows for the treatment of cancer. The majority of studies targeting cancer metabolism aims to inhibit several steps of glycolysis. So far, less explored options to interfere with mitochondrial metabolism as adjuvant therapy will be the focus of the translational WP of TRANSMIT. First of all, ACS will generate 3D cell models that recapitulate the metabolic changes leading to malignant transformation, which offer potential targets for therapeutic intervention. Recent studies on administration of biguanides, a new class of CI inhibitors, pointed out that druggability of CI function may represent a promising strategy for the urgency of new treatments for cancer. Further, treatment of melanomas with CI inhibitors and Vemurafenib elevates the anti-neoplastic potential of this BRAF-targeted drug. UNIBO will address the issue of the efficacy of pharmacological strategies either targeting CI or its downstream pathways involved in the hypoxia-driven response. UCL will be engaged in the dissection and targeting of the signaling pathways activated by mitochondrial reactive oxygen species (mtROS) and involved in the promotion of tumor metastasis. Finally, SALK will directly target the increased glycolysis and low OXPHOS activity by dietary intervention. Due to the metabolic adaptability of cancer cells, it may be necessary to combine agents that target different metabolic pathways to achieve high therapeutic efficacy. Targeting of metabolic reprogramming in cancer cells should render them more sensitive to classical chemo or radiation therapy and would likely permit reduced drug doses or duration of treatment.

Colin Wilde, ACS
Principal Investigator
UNTIL JANUARY 2020
Project: Cancer cell models to test metabolic intervention strategies

Anna Maria Porcelli, UNIBO
Project Coordinator and Principal Investigator
Project: Inducing pseudonormoxia as adjuvant therapeutic strategy for cancer
TRANSMIT Advisory Board
TRANSMIT Advisory Board guarantees that the project will fulfil all scientific and training requirements and perform at top level. The Advisory Board is composed by 3 members:
- the Scientific Publishing Industry Expert in the person of Dr. Ana Mateus (Senior Editor at Nature Cell Biology, London, UK);
- the Scientific Expert in the field in the person of Prof. Ralph DeBerardinis (Children’s Medical Center Research Institute, UT Southwestern Medical Center, Dallas, USA);
- the non-profit field Expert in the person of Dr. Helen Rippon (Chief Executive at Worldwide Cancer Research, UK).




